Introduction
As introduced in 5 featured Startups in Neurotech, BCIs can be broadly classified into three types: invasive, semi-invasive, and non-invasive. The "invasive" type of BCI introduced here is a technology that increases the possibilties of devices by implanting electrodes directly into the brain through surgery.
Currently, there are more than 1,500 neurotech-related companies listed on NeurotechX, but only 45 of them are actually involved in the application of invasive BCIs.
Among the invasive BCI companies, Neuralink leads the market in terms of size and popularity, but there are many companies that have been providing BCIs using this technology long before them.
In this article, we will introduce some of the startups that have made significant progress in their technology and vision to compete with Neuralink, along with their remarkable features and visions.
Neuralink
Before explaining Neuralink's competitors, I would like to first introduce Neuralink's value proposition and technical background that is creating a lot of buzz in this market.
The company recently announced that it has raised an additional $205M in Series C funding, and more remarkable is its investment team. Vy Capital is the lead investor, with six other firms including Google Ventures. As for angels, Sam Altman (Chairman of YC Group and CEO of OpenAI ), Fred Ehrsam (Co-founder of Paradigm and Coinbase), Ken Howery(Co-founder of PayPal and Founders Fund), and other investors who have a strong relationship with Neuralink's co-founder, Elon Musk.
These investors are accelerating Neuralink's technology R&D, and in turn, are bringing Neuralink's vision closer to reality.
The company's vision is to provide BCIs to help people with paralysis, and to expand our abilities and our world. According to Shivon Zills, Director of Operations at the company, they are thinking of 2033 - 2036 as a guideline for providing devices to healthy humans.
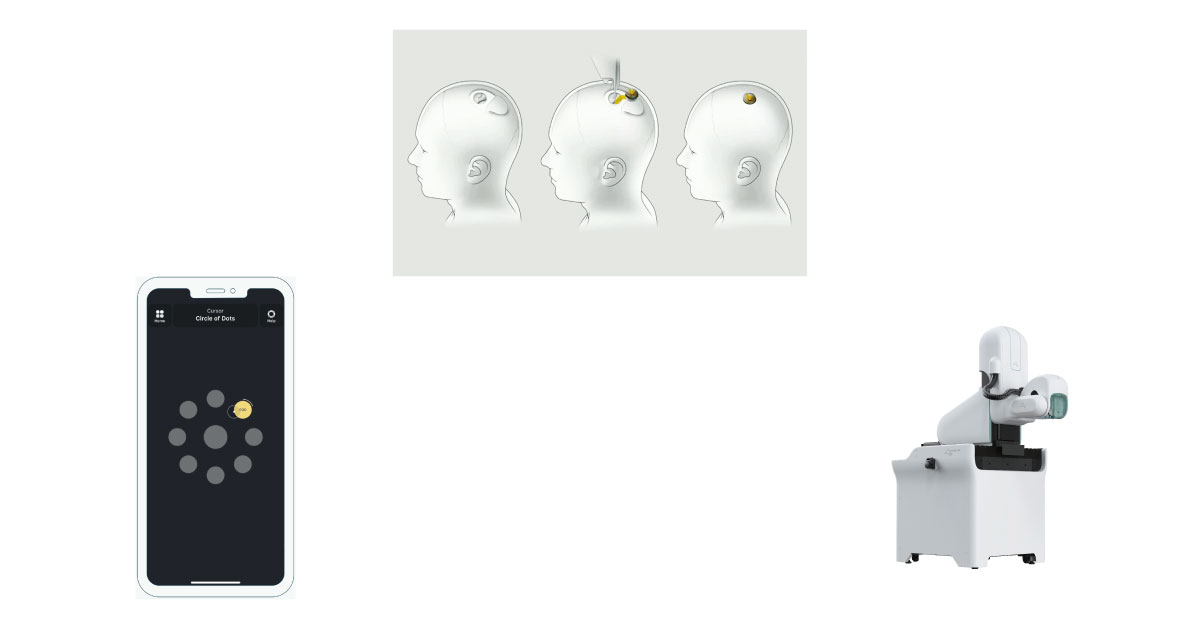 Image: Neuralink app, device, robot
Image: Neuralink app, device, robot
It is no exaggeration to say that Neuralink is a leader, not only in popularity but also in their technology.
By implanting Link, a device about the size of a coin, into the brain, it is possible to control a smartphone by simply thinking about it through mobile apps provided by the company. Remarkably, the company has also developed a robot to perform the implantation procedure automatically. By using a robot to perform the surgery instead of humans, the accuracy, reliability, and efficiency of the surgery can be guaranteed, and Neuralink devices can be provided to more people.
| Industry | Neuroscience Research |
| Headquarters | Fremont, California, USA. |
| Founded Date | 2016 |
| Employees | 101-250 |
| Funding Status | Series C |
| Total Funding | $363M |
| Investors | 13 |
Reference: Crunchbase Neuralink
Paradromics
Paradromics is a startup that provides a device called "Connexus" to restore communication in patients who have lost the ability to speak due to severe paralysis.
 Image: Connexus
Image: Connexus
The company raised $20M in seed funding in July of this year, and its technology and growth have positioned itself as a major competitor to Neuralink.
With this funding, the company will implement the feature to convert bioelectrical signals into digital signals for computers by using brain-implantable microwires connected to a device in the chest.
Last year, the company implanted more than 30,000 electrodes in the cerebral cortex of sheep to perform the biggest brain activity experiment ever. The recordings enabled high-fidelity observation of sheep brain activity in response to sound stimuli, demonstrating the effectiveness of Connexus.
Matt Angle, a co-founder of the company, describes his vision for the future.
We are combining the best of neuroscience and medical device engineering to create a robust and reliable platform for new clinical therapies.
The company plans to partner with major healthcare companies to develop new treatments for brain-related diseases.
| Industry | NeuroTech, Healthcare, Medical Device |
| Headquarters | Austin, Texas, USA. |
| Founded Date | 2015 |
| Employees | 11-50 |
| Funding Status | Seed |
| Total Funding | $49.4M |
| Investors | 15 |
Reference: Crunchbase Paradromics
Synchron
Synchron is a startup that offers a small device called "Stentrode™," implanted in the blood vessels for patients with paralysis. The implantation requires about two hours of surgery, but the device is inserted through a blood vessel in the neck and moved within the blood vessel to the location of the brain, so it can be implanted without brain surgery.
The company, like Neuralink and Paradromics, offers control of a computer with just a thought using its invasive BCI, but it is ahead of other companies in terms of speed to commercial use.
In order to sell a device that works on the human body in the U.S., the company needs to demonstrate its capabilities and safety to the FDA and get approval. The company was approved by the FDA to conduct clinical trials in July of this year. This is the first time that a commercial invasive BCI company has been approved for use on humans, and it is a step ahead of other competitors such as Neuralink, which has yet to receive approval from the FDA.
Later this year, the company plans to conduct a clinical trial of Stentrode™ in six subjects at a hospital in New York.
The company has already begun clinical trials with four patients in Australia, where it has confirmed that it can "transfer data from the motor cortex of the brain through electrodes to control digital devices."
| Industry | NeuroTech, Medical Device, Manufacturing |
| Headquarters | Campbell, California, USA. |
| Founded Date | 2016 |
| Employees | 11-50 |
| Funding Status | Series B |
| Total Funding | $50M |
| Investors | 13 |
Reference: Crunchbase Synchron
iota Biosciences
iota biosciences is a startup that provides millimeter-sized, ultrasound-driven invasive devices. The company was founded by Jose Carmena, a professor of electrical engineering and neuroscience at UC Berkeley. It is the world's first BCI device small enough to be called "neural dust", and its functionality is attracting a lot of attention.
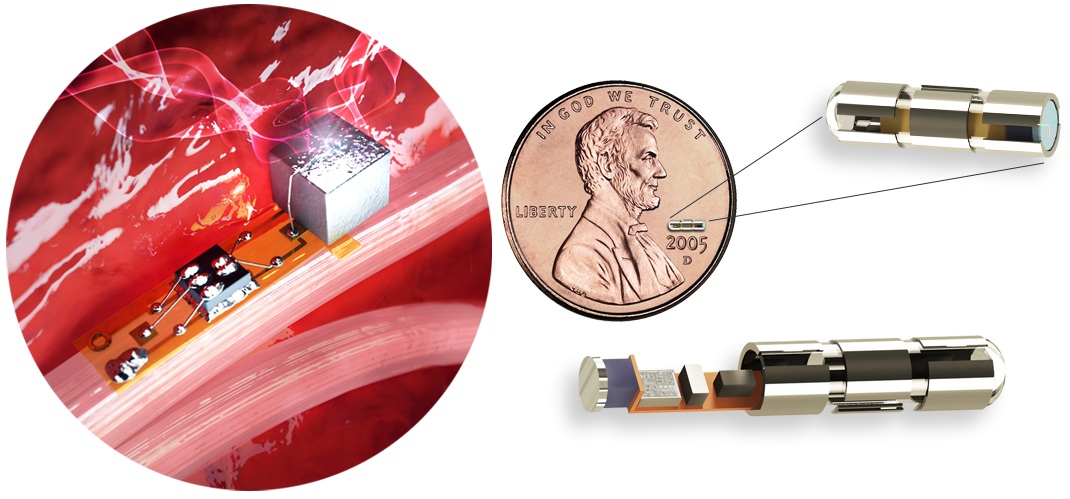 Image: iota biosciences device
Image: iota biosciences device
The key points of the device are its size and ultrasound-based functionality.
The company's small device can be implanted anywhere in the body, with the capability of capturing and stimulating the central nervous system. This will allow doctors to treat diseases such as arthritis and cardiovascular disease more effectively.
In addition, data acquisition and other interactions with the device will be done using ultrasound. Therefore, unlike the devices offered by Neuralink and the other companies mentioned above, it does not require batteries or wires.
The company was acquired by Astellas Pharma in 2020 for $127.5 million. Founder Jose Carmena and his team say that by working together, they can bring innovative new approaches to the management and treatment of diseases that affect hundreds of millions of people worldwide.
| Industry | Biotechnology |
| Headquarters | Berkeley, California, USA. |
| Founded Date | 2017 |
| Employees | 11-50 |
| Funding Status | Series A |
| Total Funding | $15M |
| Investors | 7 |
Reference: Crunchbase Iota biosciences
NeuroPace
NeuroPace is a startup that offers invasive BCI as a treatment for neurological conditions such as epilepsy. The company's device RNS® System monitors brain waves, recognizes a patient's unique "seizure onset footprint," and automatically generates small amounts of electrical signals before symptoms occur. The RNS® System is the only FDA-approved device that responds to abnormal EEG signals to treat epilepsy, and has been clinically tested on 191 patients at 31 epilepsy centers to demonstrate its safety and efficacy.
The device is implanted in the brain for approximately eight years, and experimental data showed that at the eight-year mark, the device reduced seizures by an average of 73%. In addition, out of this group, 30% of patients achieved 90% seizure reduction or better in the most recent three months, and 29% had at least one period of 6 months or more without seizures.
| Industry | Healthcare, Medical device |
| Headquarters | Mountain View, California, USA. |
| Founded Date | 1997 |
| Employees | 101-250 |
| Funding Status | Grant |
| Total Funding | $247.3M |
| Investors | 16 |
Reference: Crunchbase Neuropace
Conclusion
In this article, we introduced five startups that have applied invasive BCI technology.
Unlike non-invasive BCIs, invasive BCIs require intensive testing, often starting with multiple interations in animals before use in humans. The access to resources to develop these technologies only exist in top institutions and a handful of well-funded companies. However, several companies, led by Neuralink, are exploring the market with their abundant financial resources and innovative technologies.
With their success, there may be future where implanting devices in the brain will bring innovative experiences. On the other hand, as the FDA is regulating the testing and commercialization of devices, there will be more concern over the ethical aspects of "manipulating the brain".
The team at NeurotechJP are keeping a close eye on commercial invasive BCIs, and the impact they will have in the neurotech and medical fields.